Novel Antiviral Drug Combinations Demonstrate COVID-19 Therapeutic Potential
About the Study
Journal: Communications Biology, an open access journal from Nature Portfolio
The study is titled “Combination of antiviral drugs inhibits SARS-CoV2 polymerase and exonuclease and demonstrates COVID-19 therapeutic potential in viral cell culture.”
Authors are: Xuanting Wang1,2,11, Carolina Q. Sacramento3,4,11, Steffen Jockusch1,5,11, Otavio Augusto Chaves3,4, Chuanjuan Tao1,2, Natalia Fintelman-Rodrigues3,4, Minchen Chien1,2, Jairo R. Temerozo6,7, Xiaoxu Li1,2, Shiv Kumar1,2, Wei Xie8, Dinshaw J. Patel8, Cindy Meyer9, Aitor Garzia9, Thomas Tuschl9, Patricia T. Bozza3, James J. Russo1,2, Thiago Moreno L. Souza3,4 & Jingyue Ju1,2,10
- Center for Genome Technology and Biomolecular Engineering, Columbia University
- Department of Chemical Engineering, Columbia University
- Laboratory of Immunopharmacology, Oswaldo Cruz Institute (IOC), Oswaldo Cruz Foundation (Fiocruz), Rio de Janeiro
- National Institute for Science and Technology for Innovation on Diseases of Neglected Population (INCT/IDN), Center for Technological Development in Health (CDTS), Oswaldo Cruz Foundation (Fiocruz), Rio de Janeiro
- Department of Chemistry, Columbia University
- Laboratory on Thymus Research, Oswaldo Cruz Institute (IOC), Oswaldo Cruz Foundation (Fiocruz), Rio de Janeiro
- National Institute for Science and Technology on Neuroimmunomodulation (INCT/NIM), Oswaldo Cruz Institute (IOC), Oswaldo Cruz Foundation (Fiocruz), Rio de Janeiro
- Laboratory of Structural Biology, Memorial Sloan-Kettering Cancer Center
- Laboratory of RNA Molecular Biology, Rockefeller University
- Department of Molecular Pharmacology and Therapeutics, Columbia University
This work was supported by the Jack Ma Foundation, a gift from Columbia Engineering Member of the Board of Visitors Dr. Bing Zhao, and Fast Grants (to Jingyue Ju), the Maloris Foundation and the Memorial Sloan- Kettering Core Grant (P30CA008748) (to Dinshaw J. Patel), a grant from The JPB Foundation to Rockefeller University (to Thomas Tuschl). Funding was also provided by Conselho Nacional de Desenvolvimento Cientifico e Tecnologico (CNPq, 441019/2020-0, 307162/2017-6), Fundacao de Amparo a Pesquisa do Estado do Rio de Janeiro (FAPERJ, E-26/210.182/2020, E-26/201.067/2021, E-26/210.112/2020) and Coordenacao de Aperfeicoamento de Pessoal de Ni vel Superior -Brasil (CAPES) - Finance Code 001 (to Thiago Moreno L. Souza and Patricia T. Bozza). CNPq, CAPES and FAPERJ also support the National Institutes of Science and Tech-nology Program (INCT-IDPN, 465313/2014-0). Oswaldo Cruz Foundation/FIOCRUZ supports this study under the auspices of the Inova Program (B3-Bovespa funding, VGPDI-032-ARVC-20) (to Thiago Moreno L. Souza).
The authors declare no competing interests.
New York, NY—March 4, 2022—Researchers from Columbia Engineering, Fiocruz’s Center for Technological Development in Health and the Oswaldo Cruz Institute in Brazil, Memorial Sloan Kettering Cancer Center, and Rockefeller University recently reported that, by combining inhibitors of polymerases and exonucleases--enzymes that allow SARS-CoV-2 to reproduce--they were able to reduce SARS-CoV-2 replication 10 times more than when using just the polymerase inhibitors. They also identified a polymerase inhibitor with a unique modification that largely resists its removal from the RNA by the exonuclease. Their findings from both the molecular and cellular levels reveal the great potential of novel antiviral drug combinations to stop the spread of COVID-19 and other coronavirus diseases. The study was published February 22 by Communications Biology, an open access journal from Nature Portfolio.
“COVID has created an unprecedented public health crisis, with severe effects on the global economy and infrastructure; however, we can use the power of science to stop this pandemic,” said the Columbia team leader Jingyue Ju, Samuel Ruben-Peter G. Viele Professor of Engineering; professor of chemical engineering and pharmacology; and director, Center for Genome Technology & Biomolecular Engineering. “We expect drug combinations like the ones we found will powerfully inhibit RNA viruses such as SARS-CoV-2 and other coronaviruses that could lead to future pandemics. Because polymerase and exonuclease are highly conserved enzymes in coronaviruses with very rare mutations appearing in variants, we anticipate that therapeutics developed to target these enzymes should be widely applicable to all coronaviruses with the potential to cause serious disease.”
SARS-CoV-2, the coronavirus causing the global COVID-19 pandemic, uses a protein called polymerase to replicate its RNA genome inside infected human cells. In theory, terminating the polymerase reaction should stop the propagation of the coronavirus, leading to its eradication by the human host’s immune system. However, SARS-CoV-2 has two key enzymes that allow it to replicate: the polymerase which reproduces its RNA and a proofreading exonuclease that corrects errors in the replication process.
The presence of the exonuclease for proofreading is unique to the coronaviruses and is needed to reduce the number of mutations and thereby maintain the integrity and function of the large RNA genomes of coronaviruses. Thus, the vaccine approach has been quite effective in containing the COVID-19 pandemic because the coronaviruses do not mutate as frequently as influenza virus and HIV, which have no proofreading function and therefore mutate more frequently.
Nucleotide-based viral polymerase inhibitors are very successful drugs for treating HIV and hepatitis viruses B and C infections. However, because of the presence of the proofreading exonuclease in SARS-CoV-2, which can remove these inhibitors from the RNA, the polymerase inhibitor Remdesivir, the sole FDA-approved drug for COVID-19, is not as effective as hoped for in preventing serious disease. If the exonuclease could be concurrently inhibited or its activity evaded, viral replication would be more efficiently blocked.
“We expect drug combinations like the ones we found will powerfully inhibit RNA viruses such as SARS-CoV-2 and other coronaviruses that could lead to future pandemics.”
The research team, led by Ju and Dr. Thiago Souza, Full Researcher at the Oswaldo Cruz Institute’s Center for Technological Development in Health, decided to investigate whether the combination of polymerase and exonuclease inhibitors could work together to inhibit replication of SARS-CoV-2 more effectively, or if polymerase inhibitors with certain modifications could resist removal by the exonuclease. The Columbia Engineering team conceived the overall project and performed the molecular-level studies to identify interactions among the inhibitors and enzymes, using a novel mass-spectrometry-based approach. The Brazilian team designed and conducted the cellular studies to measure the inhibitory effects of drug combinations on virus reproduction. Dr. Thomas Tuschl’s group at Rockefeller University and Dr. Dinshaw Patel’s team at Memorial Sloan Kettering Cancer Center produced the polymerase and exonuclease complexes used in the molecular studies.
Souza’s group demonstrated that the polymerase and exonuclease inhibitors work together to block the virus’s ability to reproduce in infected lung cells. “While these results were obtained in a cell culture model, we purposely chose inhibitors already approved as drugs for treatment of other common virus infections, such as those caused by HIV and hepatitis viruses, with the aim of being able to quickly advance them to clinical trials,” Souza noted.
The team is now exploring whether the enhanced antiviral effects of the combination drugs can be demonstrated in a COVID-19 animal model, with acceptable pharmacological properties. If the results are positive, these drugs can be moved rapidly to clinical trials as they have been previously approved for treatment of other viral infections. They have also established an initiative with a consortium of pharmacologists, virologists, medicinal chemists, and structural biologists to develop new therapeutics with enhanced potency and safety profiles for COVID-19 based on the discoveries reported in this study.
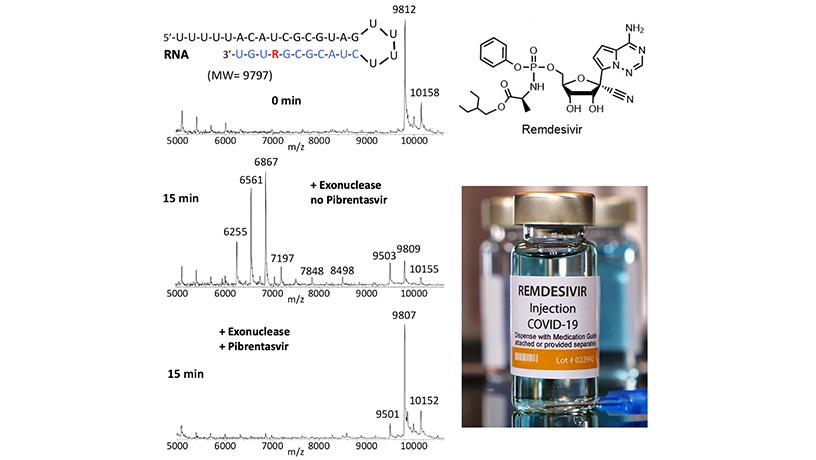
Remdesivir (R), once incorporated into RNA (top panel), is rapidly removed by SARS-CoV-2 exonuclease (middle panel); however, in the presence of the exonuclease inhibitor Pibrentasvir, the incorporated Remdesivir is largely protected from excision (lower panel). These results were obtained using a mass spectrometry assay.
The upper panel shows the RNA containing Remdesivir (red R) along with an image of a mass spectrum, showing intact RNA as the main peak. In the middle panel, the RNA is treated with exonuclease, which results in removal of nucleotides and Remdesivir from the RNA, as indicated by smaller RNA fragment peaks and the loss of the intact RNA peak. However, in the presence of Pibrentasvir, which inhibits the exonuclease, the RNA remains intact (lower panel), with the mass spectrum resembling that in the upper panel. This result might explain why Remdesivir by itself has limited efficacy for COVID-19 because of its rapid removal by the viral exonuclease. However, when the exonuclease is inhibited, because the Remdesivir remains, a more potent effect on viral replication is observed, as described in the publication.